UltraPlasma™ Xanthelasma Palpebrarum Removal
withOut! Drugs, Industrial Chemicals, Medicines, Surgery, Supplements, and Lasers.
TREATMENTSAESTHETICSBEAUTY
MedicaLabs, Ltd. | https://medicalabs.com
5/8/20243 min read
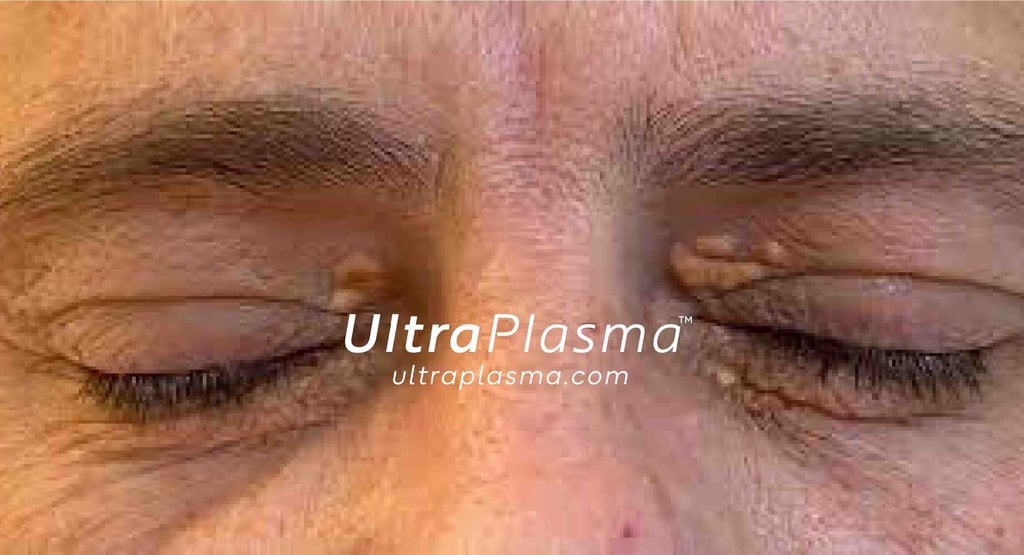

Xanthelasma Palpebrarum Removal via UltraPlasma™ Multi-Platform Plasma Systems: An Academic, Anatomical, and Engineering Perspective
Abstract
Xanthelasma Palpebrarum (XP) is a common periorbital condition characterized by yellowish cholesterol-rich plaques on the eyelids. Traditional removal methods include excision, chemical peels, cryotherapy, and lasers, often with recurrence or adverse effects. This article presents a novel, minimally invasive approach using the UltraPlasma™ multi-platform plasma system, which integrates arc, argon, and helium plasma technologies. These modalities target specific skin layers (epidermis, dermis, hypodermis) and employ reactive gas interactions (ozone, nitric oxide, reactive oxygen/nitrogen species) to remodel tissue, reduce lipid accumulations, and stimulate regeneration. The article includes anatomical analysis, plasma-tissue interaction engineering, treatment mechanisms, and clinical figure illustrations.
1. Introduction
Xanthelasma palpebrarum (XP) is a form of cutaneous xanthoma characterized by lipid-laden histiocytes in the dermis, often presenting as flat, yellowish lesions on the medial canthi. While benign, the cosmetic disfigurement prompts treatment. Existing methods can lead to pigmentary changes, scarring, or recurrence. UltraPlasma™ offers a non-contact, controlled-depth, and multi-gas plasma-based alternative, showing promising results in lesion clearance and tissue normalization with minimal side effects.
2. Anatomical and Pathophysiological Basis of Xanthelasma
2.1 Skin Layers Involved
Epidermis: Shows hyperplasia, often with thinning above XP regions.
Dermis: Contains foamy macrophages (xanthoma cells) infiltrated around capillaries.
Hypodermis: Generally spared, but plasma penetration may modulate perivascular lipid metabolism indirectly.
2.2 Cellular and Biochemical Features
Histiocytes filled with intracellular lipids, primarily cholesterol esters.
Low-grade inflammation with perivascular lymphocytic infiltration.
Poor lymphatic drainage contributes to lipid persistence.
3. UltraPlasma™ Multi-Platform System Overview
3.1 Plasma Modalities
UltraPlasma™ Arc Plasma Mode: Delivers high-energy micro-ablation to superficial and mid-epidermis. Targets plaque desiccation.
UltraPlasma™ Argon Plasma Mode: Offers mid-energy excitation, modulating dermal macrophage-lipid activity and stimulating mild fibroblast response.
UltraPlasma™ Helium Plasma Mode: Enables deep penetration (hypodermis-level) with gentle ionization, promoting lipid mobilization and lymphatic activation.
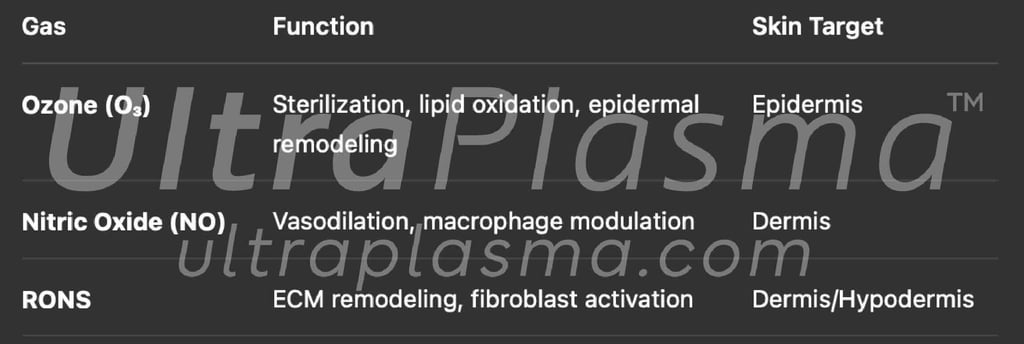
3.2 Gas-Phase Chemistry


4. Mechanism of Action in Xanthelasma Treatment
4.1 Phase 1: Superficial Ablation and Debridement
Arc plasma carbonizes superficial plaque regions.
Removes hyperkeratotic and lipid-rich layers.
Immediate desiccation reduces lesion volume.
4.2 Phase 2: Dermal Detoxification and Remodeling
Argon plasma activates dermal immune cells.
NO induces vasodilation and lipid metabolism upregulation.
RONS initiate extracellular matrix (ECM) normalization.
4.3 Phase 3: Lymphatic Mobilization and Repair
Helium plasma penetrates hypodermal zones.
Enhances drainage of lipid debris via improved microcirculation.
Stimulates local collagen production for scar-free repair.

⌘Discussion⌘
The UltraPlasma™ system introduces a multifactorial strategy to xanthelasma removal—targeting the condition’s lipid load, inflammatory base, and fibrotic risk. Unlike monomodal approaches (e.g., CO₂ laser), the combined plasma and gas interaction enhances both precision and recovery. Engineering control over frequency, energy density, and gas phase reactions enables adaptable, patient-specific therapy.
5. Engineering Parameters and Optimization
6. Clinical Protocol and Safety
6.1 Treatment Protocol
Topical anesthesia is applied (e.g., lidocaine gel).
Arc plasma is used for desiccation of surface lesions.
Followed by argon/helium sweeping over lesion and border areas.
Session time: 5–10 min per eye; 1–2 sessions recommended.
6.2 Post-Treatment Observations
Mild erythema and edema resolve in 24–48h.
Re-epithelialization occurs by Day 5–7.
Collagen remodeling continues for ~3–4 weeks.
6.3 Contraindications
Active skin infection or herpes zoster.
Blepharitis or uncontrolled diabetes (relative).






Tip-to-tissue distance: 1–3 mm
Pulse duration: 0.3–2.0 s per pulse
Spot diameter: ~0.8–1.2 mm for eyelid precision
⌘Conclusion⌘
UltraPlasma™ represents a safe, minimally invasive, and effective method for treating xanthelasma palpebrarum. The technology’s layered energy application and gas-mediated pathways provide a superior alternative to conventional methods, supporting long-term resolution with minimal recurrence and excellent cosmetic outcomes.
Shortcut for our Goals!
▼


© 2021-2026. All rights reserved.
MedicaLabs Healthcare Technologies Ltd.
https://medicalabs.com


worldwide ultraplasma.com
countries locally - coming soon:
ultraplasma.de | ultraplasma.us | ultraplasma.cn | ultraplasma.uk | ultraplasma.it | ultraplasma.fr | ultraplasma.tr | ultraplasma.co.il | ultraplasma.az | ultraplasma.ru | ultraplasma.kr | ultraplasma.es | ultraplasma.in | ultraplasma.gr | ultraplasma.cz | ultraplasma.se | ultraplasma.cl | ultraplasma.rs | ultraplasma.sg | ultraplasma.qa | ultraplasma.nl | ultraplasma.dk | ultraplasma.ro | ultraplasma.fi | ultraplasma.co.za | ultraplasma.pt | ultraplasma.al | ultraplasma.pk | ultraplasma.si | ultraplasma.ch | ultraplasma.at | ultraplasma.lt | ultraplasma.nz | ultraplasma.ae | ultraplasma.hu | ultraplasma.pl | ultraplasma.be | ultraplasma.am | ultraplasma.ar | ultraplasma.uz | ultraplasma.io | ultraplasma.info | ultraplasma.ai
