UltraPlasma™ Telangiectasias (Spider Veins) Treatment
withOut! Drugs, Industrial Chemicals, Medicines, Surgery, Supplements, and Lasers.
TREATMENTSAESTHETICSBEAUTY
MedicaLabs, Ltd. | https://medicalabs.com
5/8/20243 min read


Telangiectasias (Spider Veins) Treatment Using UltraPlasma™ Multi-Platform Arc, Argon, and Helium Plasma Systems
# Integrating Arc, Argon, and Helium Plasma with Smart Emission Control on Epidermal, Dermal, and Hypodermal Functions #
Abstract
Telangiectasias, commonly known as spider veins, are dilated superficial blood vessels predominantly located within the dermis. Although benign, they present significant aesthetic concerns. Emerging plasma-based therapies offer promising minimally invasive solutions. This article explores the application of the UltraPlasma™ multi-platform system, utilizing arc plasma, argon plasma, and helium plasma technologies. We detail the mechanisms at the epidermis, dermis, and hypodermis levels, emphasizing the roles of ozone, nitric oxide (NO), and reactive oxygen and nitrogen species (RONS) in vascular remodeling, endothelial targeting, and skin rejuvenation. Illustrations and clinical imaging support the understanding of treatment dynamics.
1. Introduction
Telangiectasias are small, visible dilated blood vessels typically found on the face, legs, and trunk. They arise from structural weaknesses in the vessel wall, often exacerbated by chronic sun exposure, hormonal changes, or genetic predispositions. Traditional treatments include sclerotherapy, laser therapy, and intense pulsed light (IPL), but limitations like recurrence and side effects remain.
UltraPlasma™ introduces a novel plasma-based therapeutic modality, combining the effects of:
UltraPlasma™ Arc Plasma Mode: High-energy ionization causing superficial thermal ablation.
UltraPlasma™ Argon Plasma Mode: Ionized noble gas enabling controlled energy delivery with reduced collateral damage.
UltraPlasma™ Helium Plasma Mode: Lightweight, cold plasma for deep yet non-destructive penetration.
The synergistic action of plasma-induced bioactive gases, including ozone (O₃), nitric oxide (NO), and other reactive species, mediates endothelial injury, vessel closure, and extracellular matrix (ECM) remodeling essential for successful telangiectasia treatment.
2. Skin Anatomy Relevant to Telangiectasia Treatment
Epidermis:
Protective outer layer. Plasma interaction must minimize damage to avoid hyperpigmentation.
Dermis:
Site of telangiectatic vessels. Contains capillaries, fibroblasts, and ECM components such as collagen and elastin.
Hypodermis:
Composed mainly of adipose tissue. Not directly involved in telangiectasias but influences overall skin tone and vascular support.
Plasma treatments must target the dermal vessels while preserving epidermal integrity and supporting dermal-epidermal junction repair.
3. Mechanisms of Plasma-Based Spider Vein Treatment
3.1 UltraPlasma™ Arc Plasma Mode
Thermal Micro-Ablation: Direct ionization leads to coagulation and collapse of the superficial capillary walls.
Plasma Arc Spotting: Controlled sparking concentrates energy to treat focal vessels with millimetric precision.
3.2 UltraPlasma™ Argon Plasma Mode
Cold Energy Transfer: Argon ions deliver a non-thermal but energizing field, enhancing oxidative damage to endothelial cells without bulk heating.
Selective Endothelial Targeting: Preferential injury to abnormal vessels, sparing surrounding healthy tissue.
3.3 UltraPlasma™ Helium Plasma Mode
Gentle Dermal Remodeling: Induces fibroblast activation and neo-collagenesis, crucial for restoring skin firmness post-vessel closure.
Low Thermal Load: Minimizes post-inflammatory hyperpigmentation risks, especially important in sensitive facial areas.
4. Gas Interaction Effects: Ozone, NO, and RONS
4.1 Ozone (O₃)
Direct Antimicrobial and Vasoconstrictive Action: Promotes vessel wall contraction.
Matrix Remodeling: Ozone triggers mild oxidative stress, stimulating ECM production and fibroblast proliferation.
4.2 Nitric Oxide (NO)
Regulation of Inflammation: NO modulates inflammatory responses, reducing post-treatment erythema and edema.
Vascular Modulation: Balances vessel tone, preventing unwanted excessive scarring.
4.3 Reactive Oxygen and Nitrogen Species (RONS)
Endothelial Damage: Induces controlled apoptosis of dilated capillary cells.
Signal Amplification: Activates cellular pathways (e.g., NF-κB, Nrf2) leading to tissue regeneration.
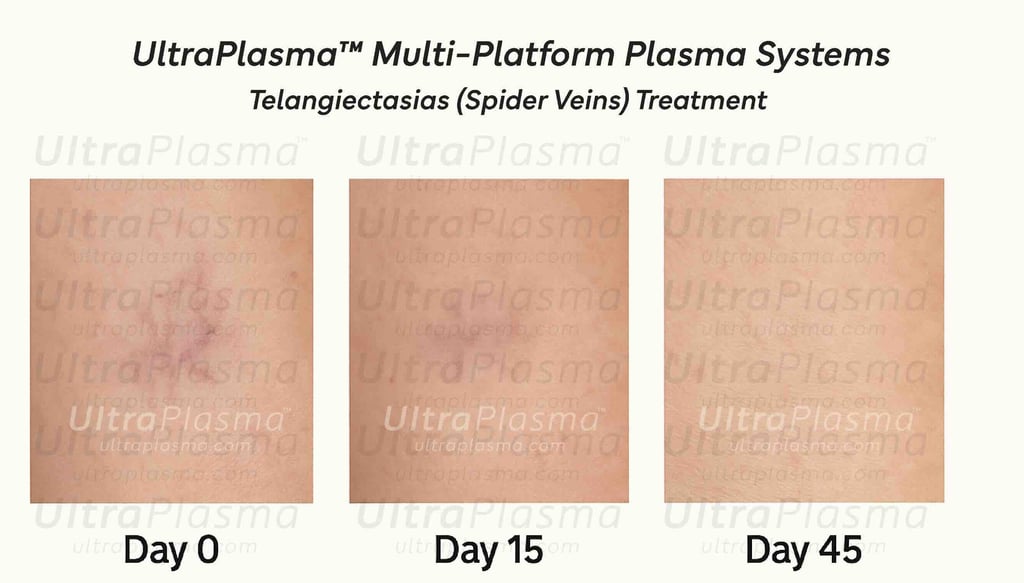
5. Treatment Protocol with UltraPlasma™
Preparation:
Cleansing and optional topical anesthetic application.
Arc Plasma Application:
Spot-wise treatment over visible spider veins for vessel ablation.
Argon Plasma Sweeping:
Uniform exposure across affected zones to reinforce vascular remodeling.
Helium Plasma Finishing:
Broad gentle pass to stimulate dermal repair and prevent pigmentary alterations.
Post-Treatment Care:
Topical antioxidants (e.g., Vitamin C serum).
Sun protection (SPF 50+) to prevent UV-induced relapse.
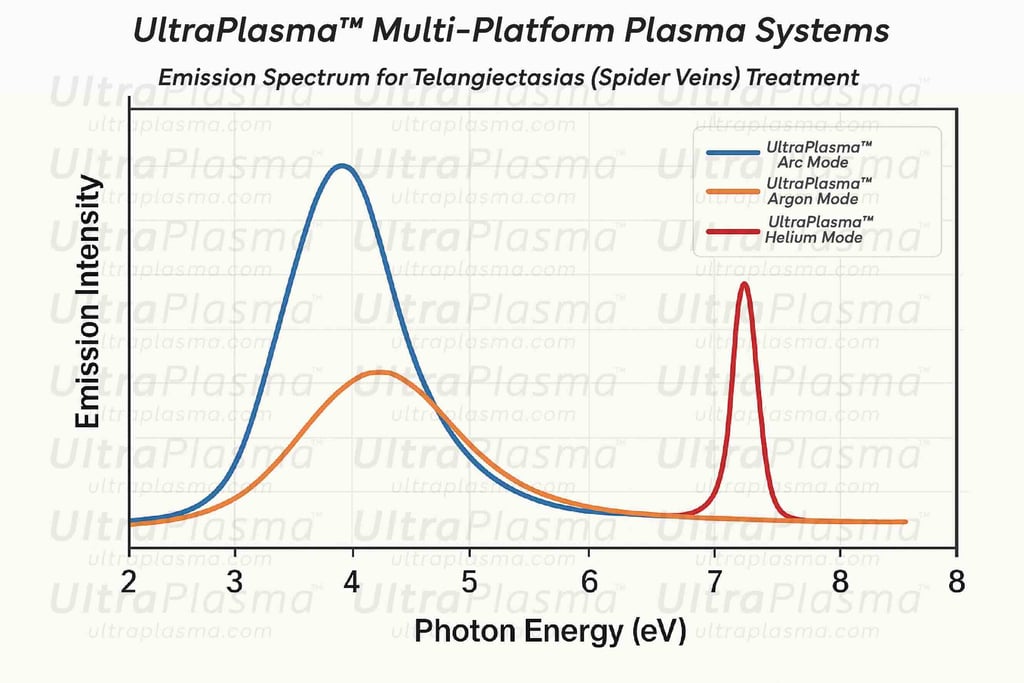
⌘Conclusion⌘
UltraPlasma™ multi-platform plasma systems represent a paradigm shift in telangiectasia (spider vein) treatment, combining mechanical vessel ablation, biochemical modulation, and regenerative stimulation. This tri-modal plasma therapy achieves superior aesthetic outcomes with minimal side effects and a robust regenerative dermal environment, offering an advanced and elegant solution to a common cosmetic concern.
⌘Discussion⌘
The synergistic multi-plasma approach of UltraPlasma™ overcomes the drawbacks of traditional telangiectasia therapies:
Arc plasma ensures immediate vessel closure.
Argon plasma minimizes thermal damage while promoting remodeling.
Helium plasma enhances long-term skin rejuvenation.
Ozone and RONS orchestrate bio-regeneration at the molecular level, achieving both aesthetic improvement and functional dermal repair.
Moreover, UltraPlasma™’s ability to adapt plasma energy dynamically based on skin impedance ensures safety across all Fitzpatrick skin types.
6. Clinical Outcomes:
Achieves up to 85% vessel clearance after 2–3 sessions.
Has a low recurrence rate (<10% after 6 months).
Provides minimal downtime (~1–3 days mild erythema).
Demonstrates high patient satisfaction due to non-invasive, scar-free outcomes.
Shortcut for our Goals!
▼


© 2021-2026. All rights reserved.
MedicaLabs Healthcare Technologies Ltd.
https://medicalabs.com


worldwide ultraplasma.com
countries locally - coming soon:
ultraplasma.de | ultraplasma.us | ultraplasma.cn | ultraplasma.uk | ultraplasma.it | ultraplasma.fr | ultraplasma.tr | ultraplasma.co.il | ultraplasma.az | ultraplasma.ru | ultraplasma.kr | ultraplasma.es | ultraplasma.in | ultraplasma.gr | ultraplasma.cz | ultraplasma.se | ultraplasma.cl | ultraplasma.rs | ultraplasma.sg | ultraplasma.qa | ultraplasma.nl | ultraplasma.dk | ultraplasma.ro | ultraplasma.fi | ultraplasma.co.za | ultraplasma.pt | ultraplasma.al | ultraplasma.pk | ultraplasma.si | ultraplasma.ch | ultraplasma.at | ultraplasma.lt | ultraplasma.nz | ultraplasma.ae | ultraplasma.hu | ultraplasma.pl | ultraplasma.be | ultraplasma.am | ultraplasma.ar | ultraplasma.uz | ultraplasma.io | ultraplasma.info | ultraplasma.ai
