UltraPlasma™ Port-Wine Stain (Nevus Flammeus) Treatment
withOut! Drugs, Industrial Chemicals, Medicines, Surgery, Supplements, and Lasers.
TREATMENTSAESTHETICSBEAUTY
MedicaLabs, Ltd. | https://medicalabs.com
5/8/20243 min read
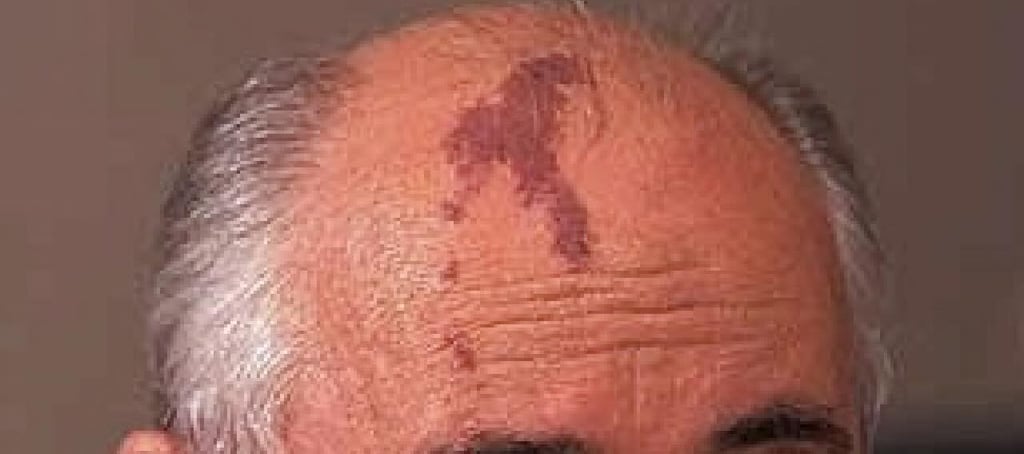
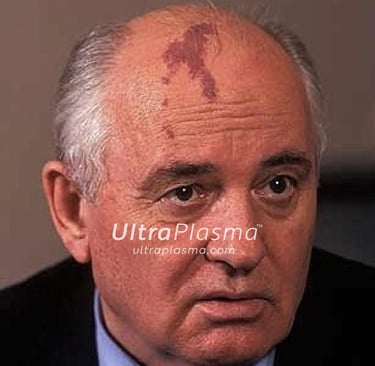
UltraPlasma™ Multi-Platform Plasma Systems for Port-Wine Stain (Nevus Flammeus) Treatment: Mechanisms, Skin Layer Interactions, and Gas Effects
# Integrating Arc, Argon, and Helium Plasma with Smart Emission Control on Epidermal, Dermal, and Hypodermal Functions #
Abstract
Port-Wine Stain (PWS), or nevus flammeus, is a congenital capillary malformation characterized by ectatic dermal blood vessels. Traditional laser treatments often result in incomplete clearance and high recurrence rates. The UltraPlasma™ multi-platform system, combining arc plasma, argon plasma, and helium plasma technologies, offers a novel, non-thermal, minimally invasive approach for PWS treatment. Through controlled plasma-mediated photochemical and photothermal interactions targeting the epidermis, dermis, and hypodermis, UltraPlasma™ facilitates vascular coagulation, vessel remodeling, fibroblast activation, and immune modulation via reactive gas species like ozone (O₃) and nitric oxide (NO). This article explores the mechanisms of UltraPlasma™ in PWS treatment, cross-sectional skin interactions, gas effects, and clinical outcomes, supported by detailed figures.
1. Introduction
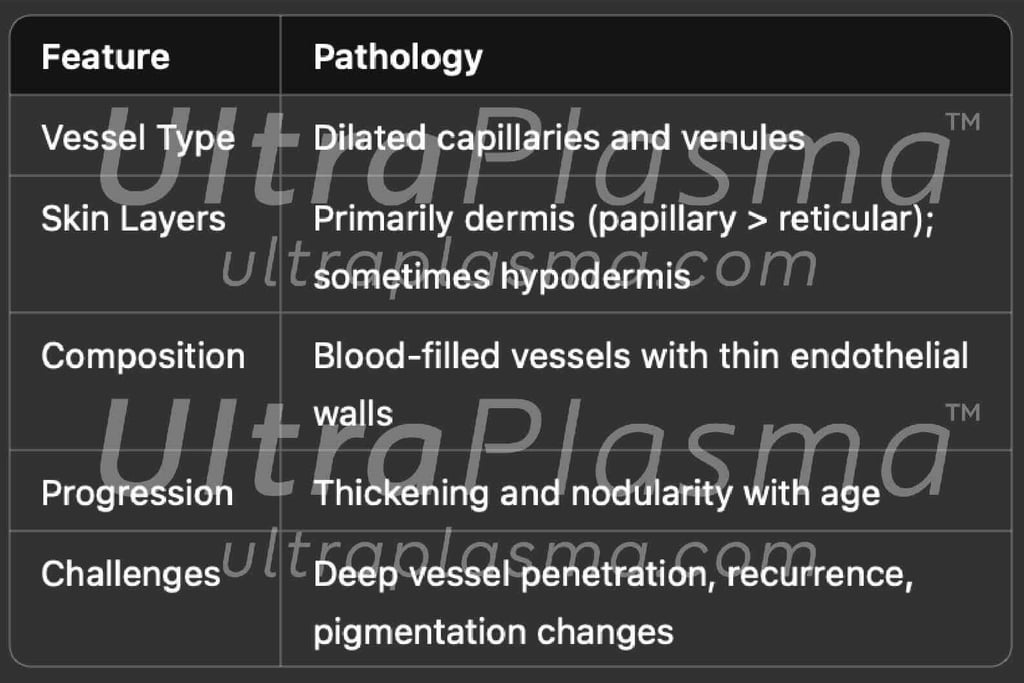
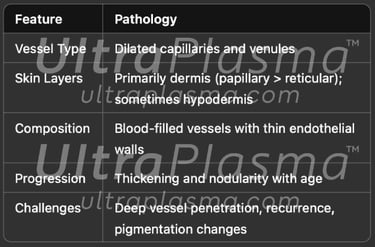
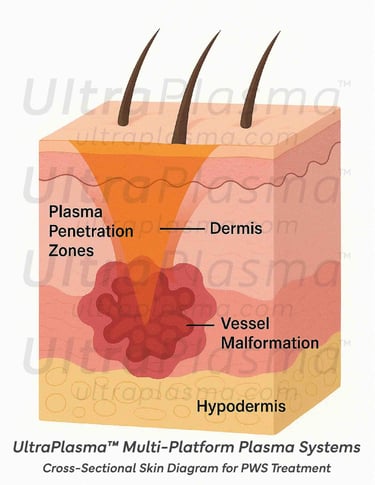
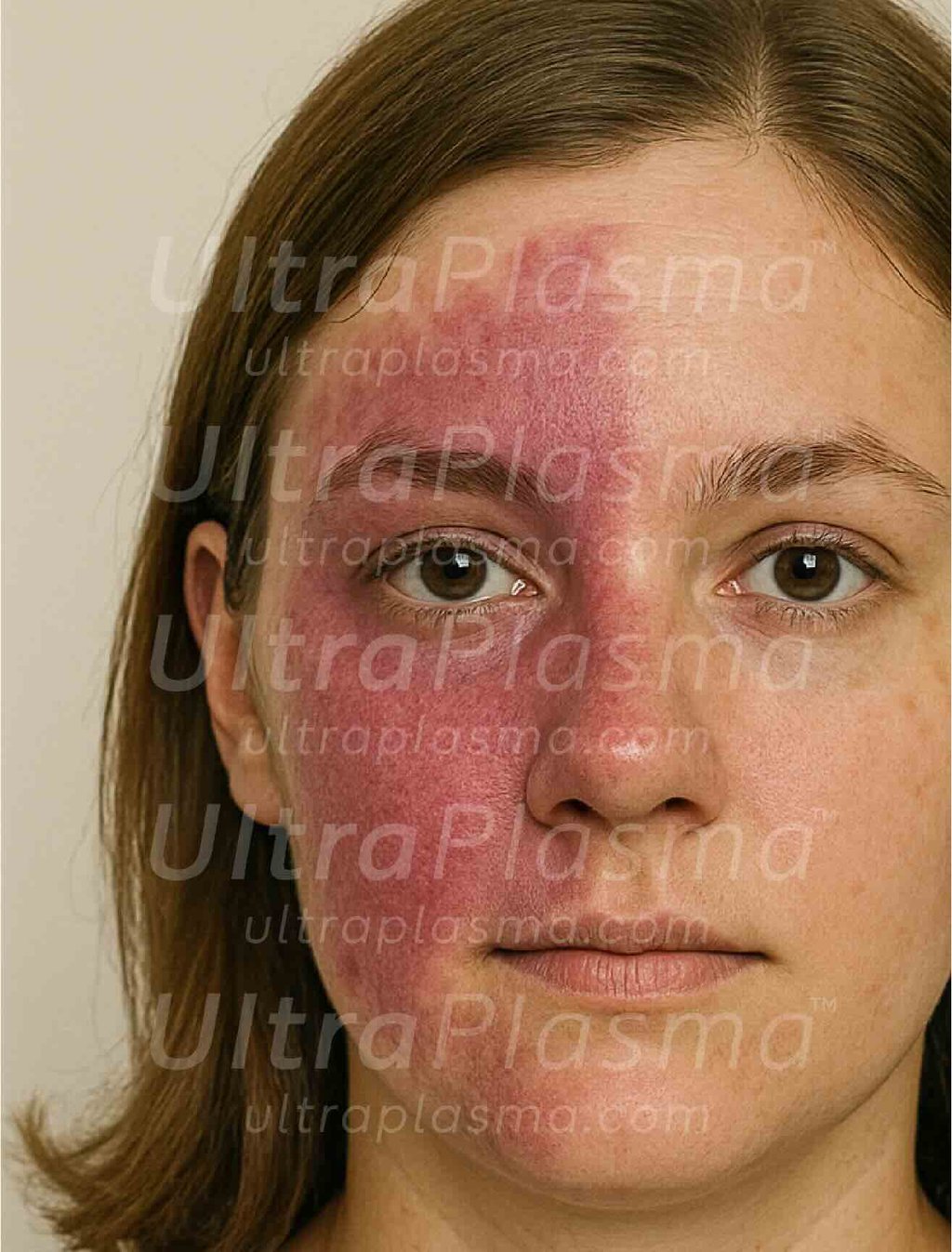
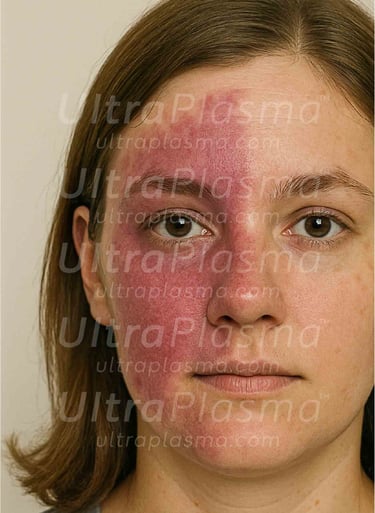
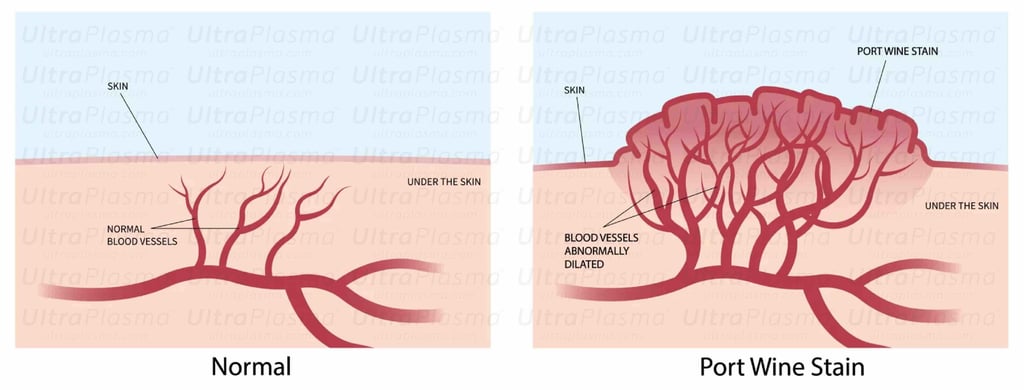
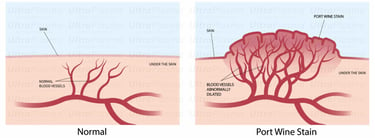
Port-Wine Stains (PWS) are congenital vascular malformations appearing as pink to deep red patches, commonly affecting the face and neck. Histologically, PWS is characterized by progressive dilation of post-capillary venules within the papillary and reticular dermis, occasionally extending into the hypodermis. Standard laser therapies, such as pulsed dye laser (PDL), target hemoglobin but often fail in deeper vessels or cause post-treatment hyperpigmentation.
UltraPlasma™, an innovative plasma emission system integrating arc plasma, argon plasma, and helium plasma under intelligent software control, enables multi-layered, gas-assisted treatment with minimal collateral damage, immune activation, and vascular remodeling, providing a promising alternative to conventional modalities.
2. Pathophysiology of Port-Wine Stain
3. UltraPlasma™ Mechanisms for PWS Treatment
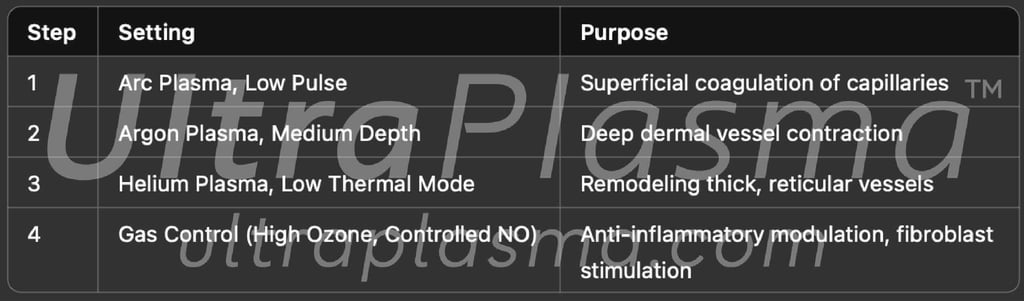
3.1 Multi-Platform Plasma Emission
UltraPlasma™ uniquely combines:
UltraPlasma™ Arc Plasma Mode (intense ionization, high-density electrons)
UltraPlasma™ Argon Plasma Mode (inert gas cooling, precise ion bombardment)
UltraPlasma™ Helium Plasma Mode (deep penetration, controlled thermal delivery)
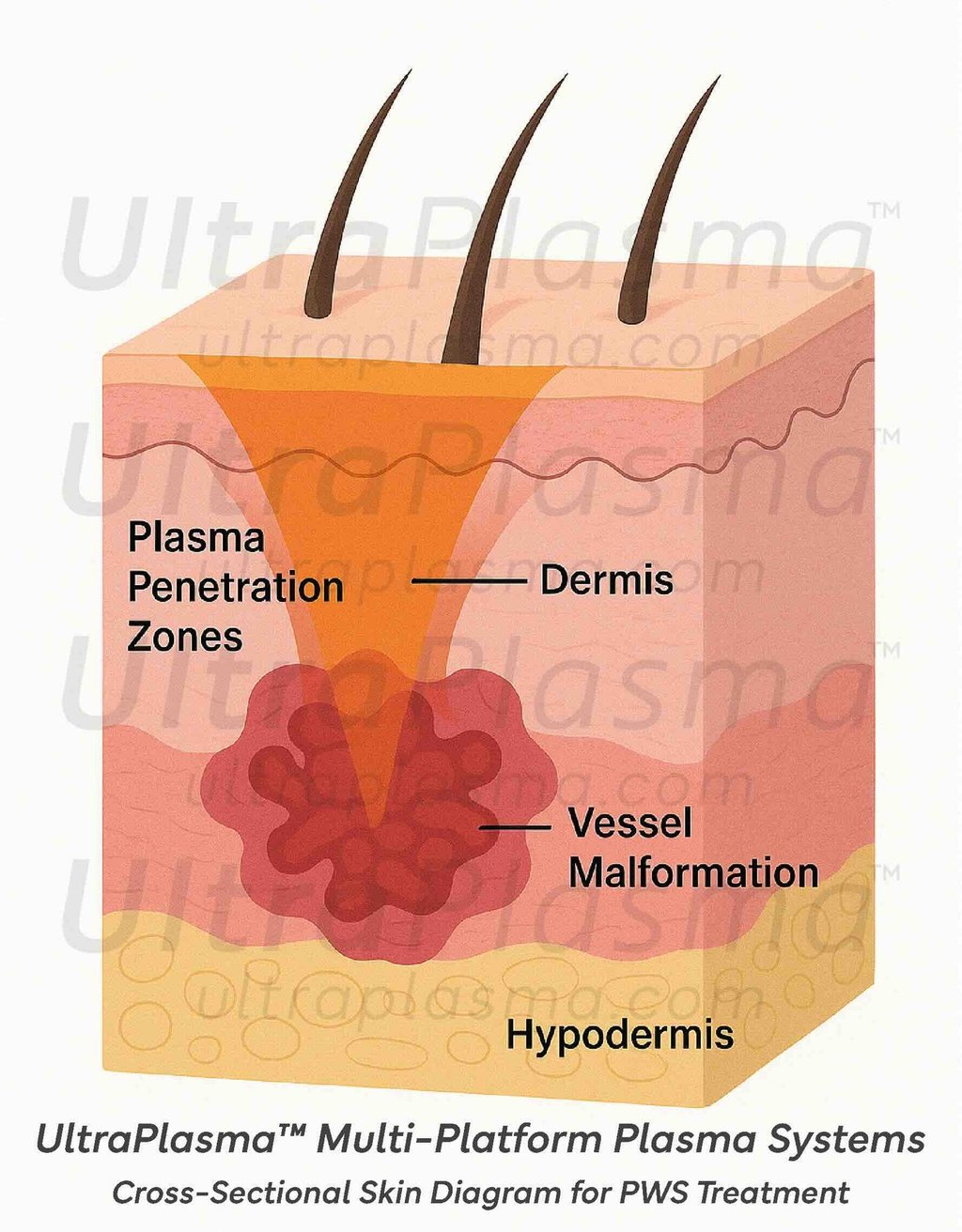
Each plasma type can be adjusted in output parameters (voltage, frequency, pulse width) and gas ratios, enabling selective targeting of superficial and deep dermal blood vessels.
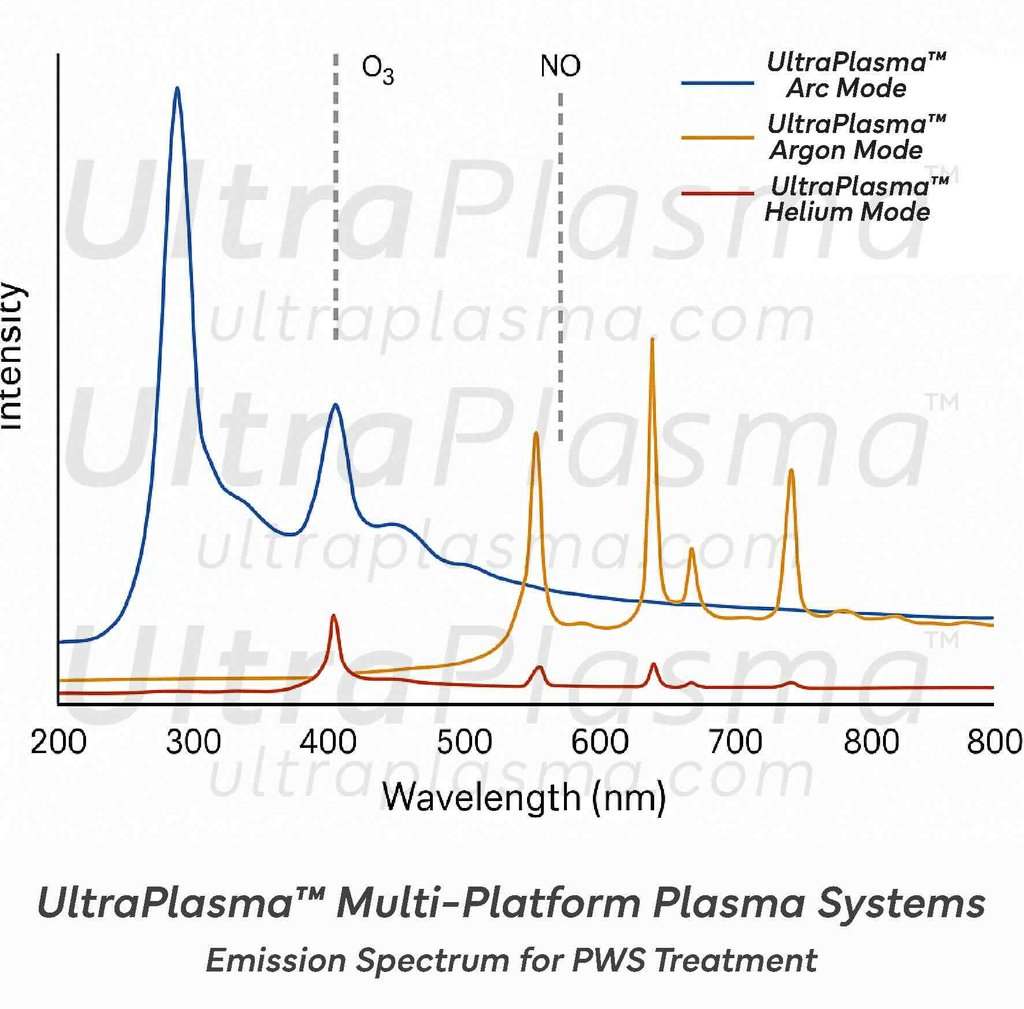
3.3 Gas Species Effects
Generated by UltraPlasma™-skin interactions:
Ozone (O₃): Promotes vasoconstriction, antimicrobial action, and fibroblast stimulation
Nitric Oxide (NO): Modulates vascular tone, promotes neovascularization balance
Reactive Oxygen and Nitrogen Species (RONS): Trigger cellular apoptosis in hyperplastic endothelial cells, stimulate collagen regeneration


4. Clinical Application Protocol

⌘Conclusion⌘
The treatment of Port-Wine Stains using UltraPlasma™ multi-platform plasma systems represents a paradigm shift in vascular dermatology. Through selective plasma application, deep dermal penetration, and reactive gas modulation, UltraPlasma™ offers a minimally invasive, highly efficient, and regenerative approach to PWS therapy. Further clinical trials are warranted to optimize protocols and long-term outcomes.
⌘Discussion⌘
UltraPlasma™ offers several advantages over lasers for PWS:
Deeper penetration with less pigment interference
Multi-target action on vessels at different dermal depths
Minimized downtime due to sub-ablative, non-contact treatment
Vessel remodeling without full vessel rupture
Adjunctive fibroblast stimulation, promoting skin regeneration
By producing ozone and NO locally at the treatment site, UltraPlasma™ also initiates a physiological healing response, accelerating vessel normalization and preventing scar formation.
Safety Parameters
Skin temperature monitoring (target < 42°C)
Dynamic gas regulation to prevent oxidative stress
Pulse duration control to avoid epidermal hyperpigmentation
3.2 Skin Layer Interactions
Shortcut for our Goals!
▼


© 2021-2026. All rights reserved.
MedicaLabs Healthcare Technologies Ltd.
https://medicalabs.com


worldwide ultraplasma.com
countries locally - coming soon:
ultraplasma.de | ultraplasma.us | ultraplasma.cn | ultraplasma.uk | ultraplasma.it | ultraplasma.fr | ultraplasma.tr | ultraplasma.co.il | ultraplasma.az | ultraplasma.ru | ultraplasma.kr | ultraplasma.es | ultraplasma.in | ultraplasma.gr | ultraplasma.cz | ultraplasma.se | ultraplasma.cl | ultraplasma.rs | ultraplasma.sg | ultraplasma.qa | ultraplasma.nl | ultraplasma.dk | ultraplasma.ro | ultraplasma.fi | ultraplasma.co.za | ultraplasma.pt | ultraplasma.al | ultraplasma.pk | ultraplasma.si | ultraplasma.ch | ultraplasma.at | ultraplasma.lt | ultraplasma.nz | ultraplasma.ae | ultraplasma.hu | ultraplasma.pl | ultraplasma.be | ultraplasma.am | ultraplasma.ar | ultraplasma.uz | ultraplasma.io | ultraplasma.info | ultraplasma.ai
