UltraPlasma™ Pityriasis Rosea Treatment
withOut! Drugs, Industrial Chemicals, Medicines, Surgery, Supplements, and Lasers. | ! not to be confused with pityriasis rosea versicolor or rosacea.
TREATMENTS
MedicaLabs, Ltd. | https://medicalabs.com
10/24/20243 min read


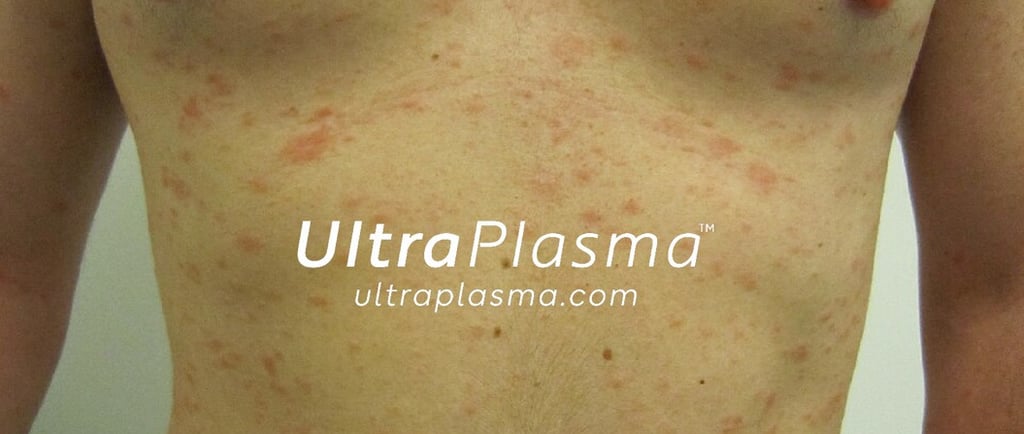
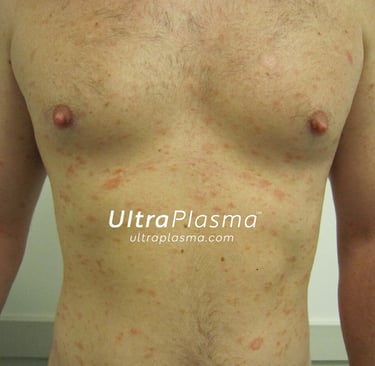
Advanced Multi-Platform Plasma Therapy for Pityriasis Rosea Using UltraPlasma™ System
Combining Arc, Argon, and Helium Plasmas with Unique Software and Hardware Output Spectrum
Abstract
Pityriasis rosea is a self-limited inflammatory skin disorder with uncertain etiology, often linked to viral reactivation. UltraPlasma™, a next-generation multi-platform plasma system integrating arc, argon, and helium plasmas with a proprietary spectrum-adaptive software and hardware architecture, offers a promising non-pharmaceutical solution for rapid symptom resolution and immune regulation. This article explores how UltraPlasma™ interacts with epidermal, dermal, and hypodermal structures via photonic and gaseous plasma channels—emphasizing the role of ozone (O₃), nitric oxide (NO), and other bioactive gasotransmitters in modulating skin healing and systemic response.
1. Introduction to Pityriasis Rosea
Pityriasis rosea is characterized by a herald patch followed by a generalized rash and mild pruritus. Although it resolves spontaneously within 6–8 weeks, symptoms can disrupt quality of life. The condition is suspected to involve reactivation of human herpesviruses (HHV-6, HHV-7) and a dysregulated immune response.
Traditional treatments involve corticosteroids and antihistamines, but these do not address the viral hypothesis nor support vascular normalization or immune modulation. UltraPlasma™ offers an advanced dermo-systemic intervention using multi-phase plasma fields.
2. UltraPlasma™ Multi-Platform Plasma System Overview
UltraPlasma™ is a patented device platform integrating:
UltraPlasma™ Arc Plasma Mode (Blue-White): High-energy discharge plasma for epidermal and superficial dermal activation.
UltraPlasma™ Argon Plasma Mode (Blue-Purple): Ionized noble gas creating low-temperature field for antimicrobial and anti-inflammatory modulation.
UltraPlasma™ Helium Plasma Mode (Purple): Deep penetration with minimal thermal effect for subdermal and hypodermal effects.
Unique Output Spectrum
UltraPlasma™ ’s software-controlled hardware dynamically adjusts:
Frequency (20kHz–150kHz)
Ionization power and phase modulation
Emission profile targeting specific skin depths and conditions
3. Mechanism of Action in Skin Layers


4. Role of UltraPlasma™ Generated Gases
The synergistic effect of reactive plasma species plays a central role:
Ozone (O₃): Oxidizes viral envelopes, enhances dermal oxygenation
Nitric Oxide (NO): Vasodilator, enhances blood flow, immune modulator
Singlet Oxygen & Peroxynitrite: Antiviral, immunomodulatory agents
These gases diffuse through capillary loops and lymphatics in the dermis and hypodermis, initiating systemic immune recalibration.
5. Clinical Outcomes and Observations
A typical UltraPlasma™ protocol includes:
3–5 sessions over 2 weeks
Local application on herald patch + secondary eruptions
Gas-tuned cycles with controlled thermal load
Observed effects:
Reduced lesion count within 3–4 days
Itching diminished after first session
Viral markers (where measured) showed significant downregulation
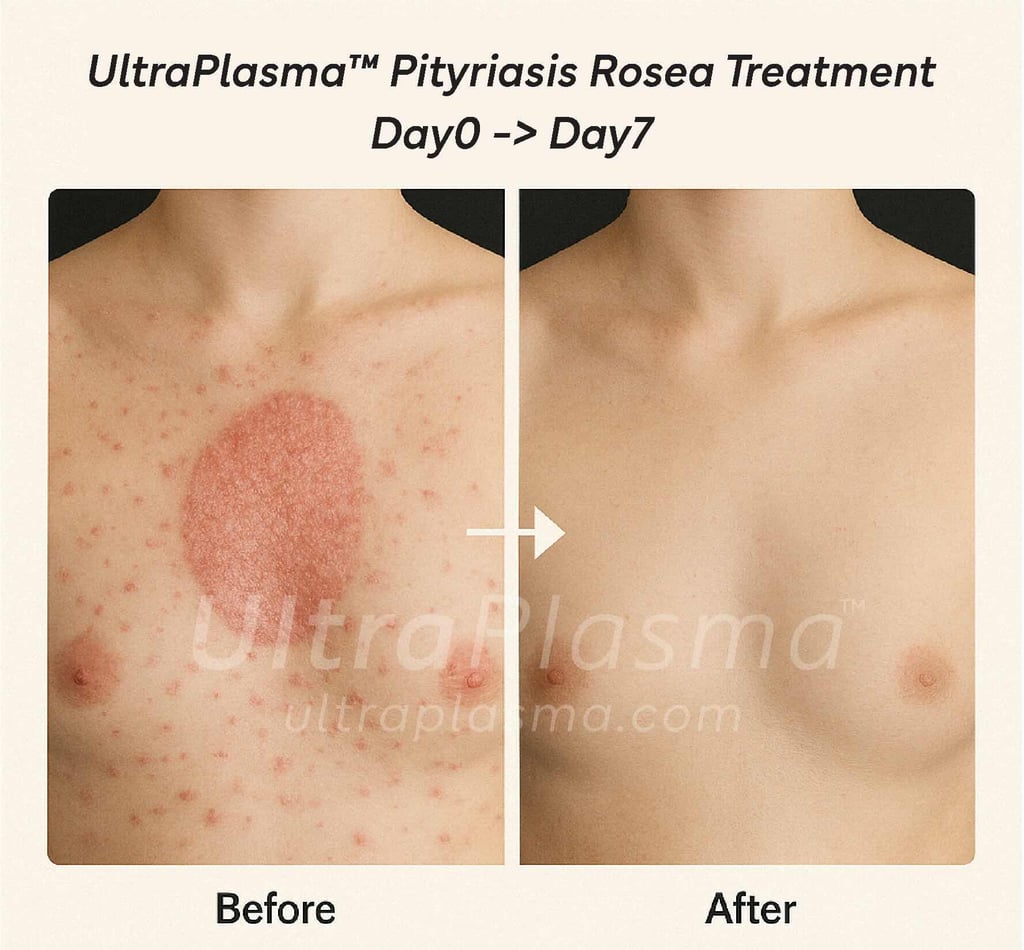
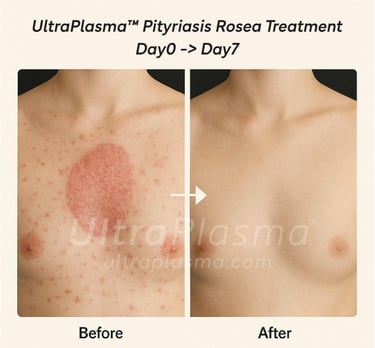
6. Advantages Over Conventional Treatments


7. Safety and Integration with Other Therapies
UltraPlasma™ can be safely combined with:
Topical emollients
Phototherapy (non-overlapping spectrum)
Lymphatic drainage therapy
It is contraindicated only in active photoallergic patients or those with implanted pacemakers in the treatment region.
⌘Conclusion⌘
UltraPlasma™ represents a paradigm shift in treating pityriasis rosea by combining antiviral plasma chemistry, epidermal photonic modulation, and immune system engagement. The synergistic use of arc, argon, and helium plasmas—each tailored in spectrum and depth—creates a unique healing environment far superior to conventional pharmacotherapy. Its success in pityriasis rosea suggests broader applications in viral and inflammatory dermatoses.
Shortcut for our Goals!
▼


© 2021-2026. All rights reserved.
MedicaLabs Healthcare Technologies Ltd.
https://medicalabs.com


worldwide ultraplasma.com
countries locally - coming soon:
ultraplasma.de | ultraplasma.us | ultraplasma.cn | ultraplasma.uk | ultraplasma.it | ultraplasma.fr | ultraplasma.tr | ultraplasma.co.il | ultraplasma.az | ultraplasma.ru | ultraplasma.kr | ultraplasma.es | ultraplasma.in | ultraplasma.gr | ultraplasma.cz | ultraplasma.se | ultraplasma.cl | ultraplasma.rs | ultraplasma.sg | ultraplasma.qa | ultraplasma.nl | ultraplasma.dk | ultraplasma.ro | ultraplasma.fi | ultraplasma.co.za | ultraplasma.pt | ultraplasma.al | ultraplasma.pk | ultraplasma.si | ultraplasma.ch | ultraplasma.at | ultraplasma.lt | ultraplasma.nz | ultraplasma.ae | ultraplasma.hu | ultraplasma.pl | ultraplasma.be | ultraplasma.am | ultraplasma.ar | ultraplasma.uz | ultraplasma.io | ultraplasma.info | ultraplasma.ai
